We’ve already written that hydrogen was generated in the seconds after the big bang in the article ‘What is hydrogen and why use it to store energy’ but how have we used hydrogen since it was discovered in the seventeenth century as a distinct element? A number of observations about the physical characteristics of hydrogen have led to it being used in many different ways over the years since it was discovered. Notably, it is extremely light, it is combustible, it can hold a lot of energy and in Earth’s atmospheric conditions it wants to bond with other elements to form chemical compounds rather than exist as a pure gas.
One of the more infamous uses for hydrogen has been to fill balloons for buoyancy. The lightness of hydrogen when compared to ground level air means that when we fill a balloon with hydrogen it will float and with enough volume, can carry large payloads to great heights. This was first achieved in the 18th century and utilised until the 20th century as a mode of transport with zeppelins most famously being used extensively for about 30 years before the Hindenburg disaster in 1937. Today, we still fill balloons with hydrogen but generally only for high altitude, unmanned balloon flights (typically weather balloons) that reach the very top of the atmosphere.

Due to it’s combustibility, hydrogen has been and is used as a fuel. All the way back in the very early 1800s, Francois Isaac de Rivas built the first internal combustion engine that was powered by hydrogen (stored in a small balloon) and oxygen not diesel or gasoline as we’re used to today. In the de Rivaz engine (pictured - hydrogen store labelled as D) a small spark was electrically generated inside a cylinder that powered a piston. Today, hydrogen powered vehicles are normally electric, with the hydrogen being used in an electro-chemical reaction in a fuel cell rather than combusted. Hydrogen fuelled fork lifts are extremely commonplace as they have to operate indoors so cannot give off pollutants, with water vapour as the only by-product. Hydrogen powered vehicles are believed to be a viable way of de-carbonising transport, particularly heavy goods vehicles, aircraft and boats.
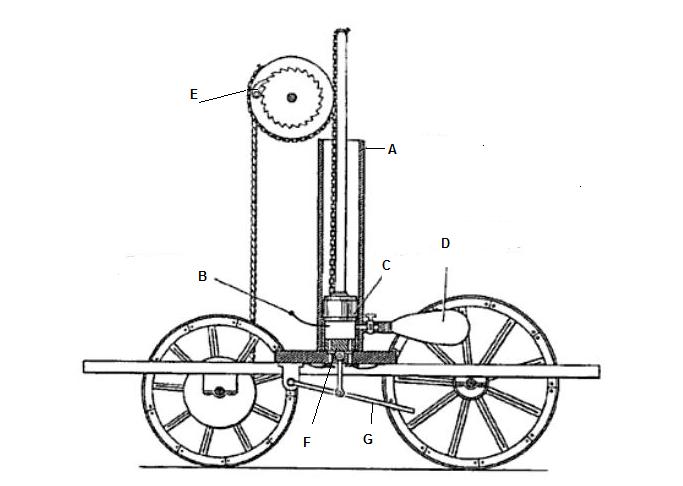
The 1807 charette of de Rivaz. A = Cylinder, B = Spark ignition, C = Piston, D = Balloon containing hydrogen fuel, E = Ratchet, F = Opposed piston with air in and exhaust out valves, G = Handle for working opposed piston.
Hydrogen is routinely used as a fuel in rockets due to having such a high energy density by weight. Liquid hydrogen is the fuel of choice for space rockets as it burns with such extreme intensity and in combination with liquid oxygen, yields the highest specific impulse (efficiency in relation to amount consumed) of any rocket propellant.
Hydrogen has also been burned in houses in the past, as it was a key constituent of town gas (a mixture of hydrogen, carbon monoxide and carbon dioxide), which was produced from gasification of coal and piped throughout the UK gas network to be used for heat and hot water before North Sea discoveries allowed us to rely on natural gas (CH4) instead.
From the 20th century onward hydrogen is used on an industrial scale, primarily for two processes. Very early in the 20th century the Haber process was invented as a way of producing ammonia (NH3) on industrial scales and is used today to generate significant amounts for fertiliser. Generating ammonia by the Haber method involves passing gaseous hydrogen and nitrogen over catalysts at high pressures and temperatures. Ammonia was also used in the production of explosives during WW1.

The other current major industrial use of hydrogen is in refining of hydrocarbons. Hydrogen is used in the cracking process of long-chain hydrocarbons and to remove sulphur content from hydrocarbons so that when they are burned they give off less sulphur oxides, which are a major pollutant – and responsible for acid rain! Hydrocracking involves introducing hydrogen gas to the long-chain hydrocarbons at high temperature, which then replace a carbon-carbon bond to create two shorter chain hydrocarbons. Hydrosulphurisation again requires the hydrocarbons to be heated in the presence of hydrogen gas as well as a catalyst, which causes the sulphur to bond with the gaseous hydrogen to form hydrogen sulphide, which is captured and typically converted into elemental sulphur or sulphuric acid.
There are and have been numerous other uses for hydrogen gas but now both of the modern industrial uses for hydrogen require a significant amount of the gas and account for the vast majority of global demand. According to the IEA, approximately 72 million tonnes of hydrogen was used in 2015, largely shared between ammonia production for fertilizers and for the desulphurisation of hydrocarbons during refining, which used a combined 68 million tonnes. This demand for hydrogen is increasing, largely due to greater environmental legislation requiring more desulphurisation and larger amounts of heavy oils being extracted, which need cracking. Most of this hydrogen is currently grey – it is produced by gas reformation of natural gas, which is a major emitter of carbon dioxide. There are many resources being committed to making the gas reformation process more environmentally friendly by capturing and storing or utilising the carbon dioxide, which is known as blue hydrogen (check out our previous article here to learn more about the colours of hydrogen).
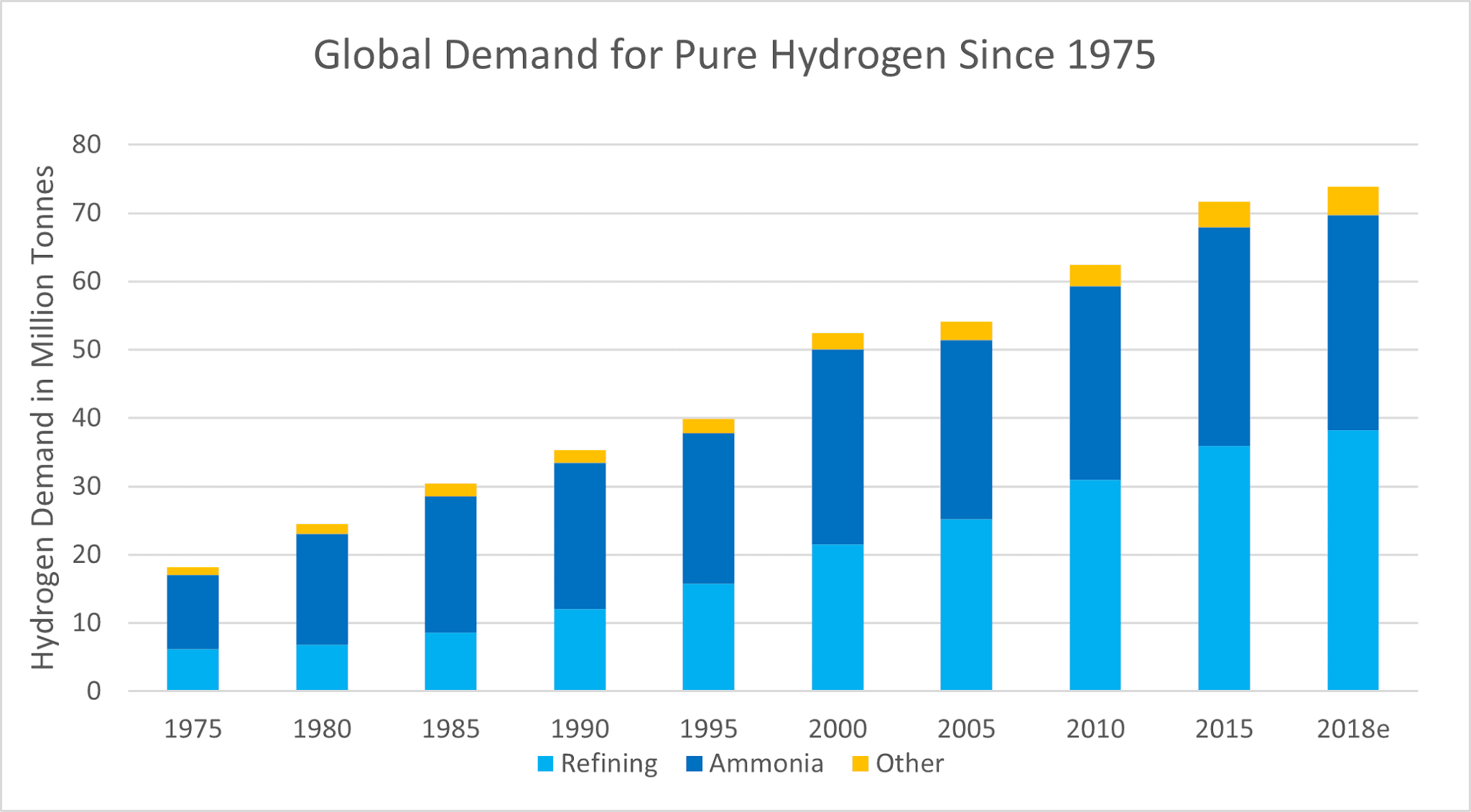
As more uses for hydrogen are introduced, primarily as a fuel for mobility and as an energy storage vector, we are going to have to produce greater quantities while taking into account the environmental impact. At HydroGenesis we truly believe that hydrogen will play a huge part in energising our lives but feel it should be generated as green hydrogen through electrolysis using renewable energy sources with blue hydrogen acting as a bridge while global electrolysis capacity is increased. Green hydrogen will become a mainstream method in time as equipment prices will become more competitive, due to scaling-up, and renewable energy sources become more abundant.

