The fuel cell is the key piece of kit in the HydroGenesis system that creates electricity when there is insufficient solar energy hitting the photovoltaic panels.
There are several types of fuel cells, depending on the fuel used and the different components and chemical processes in the fuel cell. Fuel Cells were first invented in the mid-19th century with British and German physicists making parallel advancements. The first practical hydrogen fuel cell (an alkaline fuel cell) was developed by an Englishman in 1932, which was used in the Apollo moon project and still used by NASA to this day. Hydrogen fuel cells are used in many extreme places including in the International Space Station as well as German submarines.

International space station
HydroGenesis, as our name suggests, will use fuel cells fueled by locally generated hydrogen via onsite electrolysers; but how do hydrogen fuel cells generate electricity?
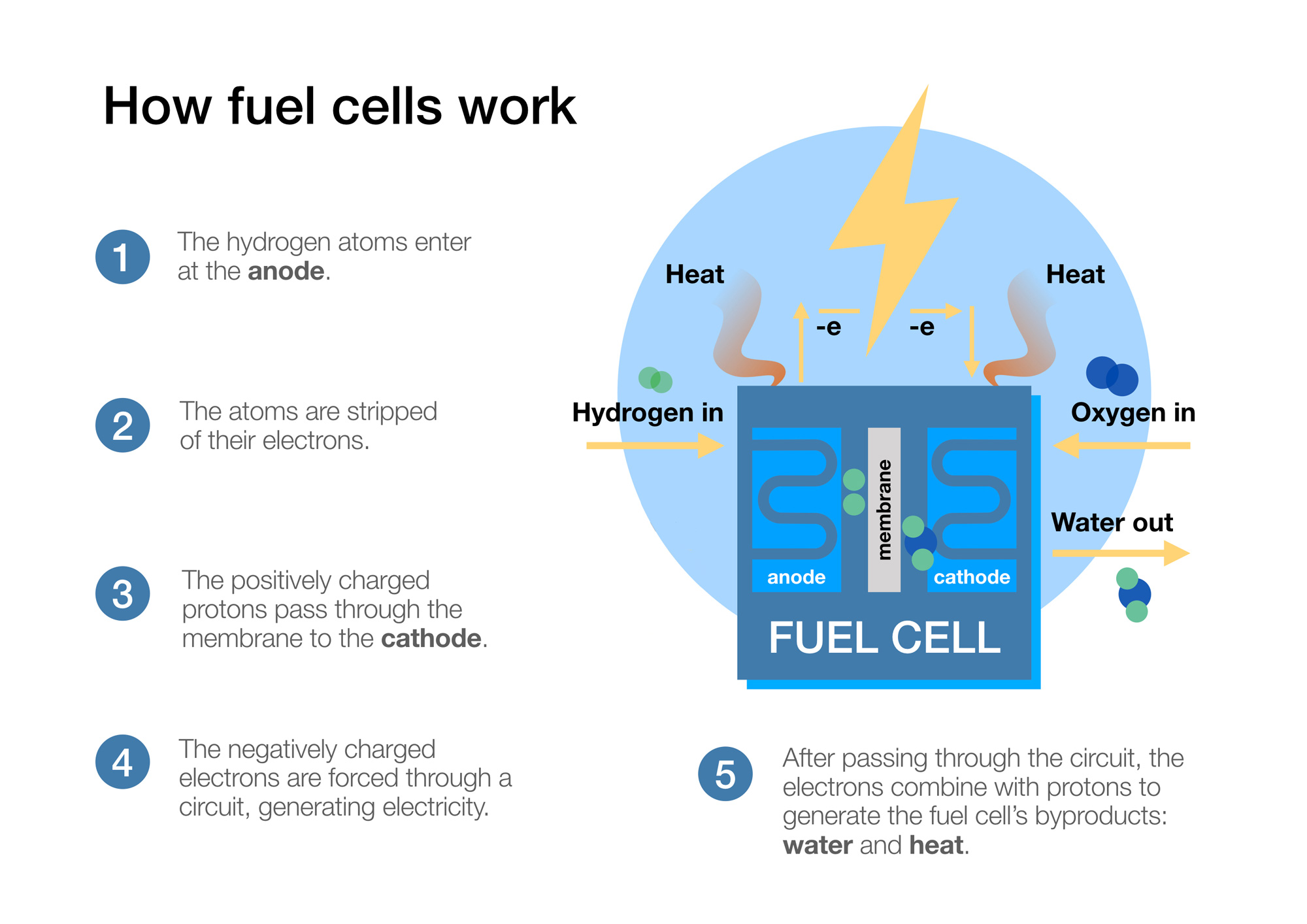
- A hydrogen fuel cell takes hydrogen in at the anode and oxygen at the cathode.
- The hydrogen atoms are stripped of their electrons (negatively charged subatomic particles) via oxidation at the anode, achieved via a catalyst, and these are forced to do “work” around an electrical circuit.
- The protons (positively charged subatomic particles) are almost 2000 times smaller than the electrons and are able to pass through the electrolyte (or a solid membrane in modern fuel cells, see more below).
- The protons and electrons reunite at the cathode and react with the oxygen in a reduction reaction which creates water (specifically 2H20).
The chemical processes for the above 4 steps can be notated as:
Oxidation Reaction at the Anode 2H2 → 4H+ + 4e−
Reduction reaction at the Cathode O2 + 4H+ + 4e− → 2H2O
It is eloquent to think of the fuel cell as the reverse process of the electrolyser which uses electricity to split water into oxygen and hydrogen.
A Proton Exchange Membrane (PEM) fuel cell converts the hydrogen into electricity via a solid proton-conducting membrane as the electrolyte. This is rapidly becoming the most popular type of fuel cell as due to lower operating temperatures and the use of non-corrosive electrolytes, which now require cheaper more abundant metals such as steel as opposed to requiring rare earth metals such as platinum for the anode.
In the fuel cells there is no combustion as the process is an electrochemical reaction, meaning they operate extremely quietly, with zero-carbon emissions, and only output water and heat other than the electricity. The process is exothermic and approximately half of the energy is transferred to heat with the electricity making up the remainder. HydroGenesis intend to make use of this heat and therefore increase the useful efficiency of the energy reaction.
Fuel cells convert chemical energy directly into electrical energy, whereas as the internal combustion engine (ICE) takes chemical energy (fossil fuels) converts it to mechanical energy to then produce electrical energy; and with each conversion there are inefficiencies, noise and heat.

Since each energy transformation involves some amount of energy loss, the fuel cell is more efficient at producing electricity than an engine or turbine. Fuel cells operate at an efficiency of electrical conversion of about 40 to 50%, a fossil-fuelled power station is typically 35% efficient, and an ICE operates at about 15% to 20% efficiency.
POWER: Hydrogen fuel cells typically produce between 0.5 V and 0.8 V per cell, but the cells can be connected in series to increase the voltage, this connection is called a “Fuel Cell Stack”. The more cells stacked, the larger the total area available to produce current:
current x voltage = power
Amps (A) x voltage (V) = Power/Watts (W)
So, multiple stacks can be combined to enable the production of the required power level for the scoped system. Both fuel cells and electrolysers are now much more economical at small scales and are stackable, allowing us to build the required system size for each application.
A disadvantage of fuel cells is that they have a latency period (ramp-up time) between reaction commencement and power output; therefore a “battery” is typically deployed to be able to deliver on demand the variable load of the electrical requirement. Batteries and fuel cells both create electricity in an electrochemical reaction, though batteries have the chemicals stored inside (such as Lithium) and Fuel cells outside in hydrogen cylinders.

Hydrogen Fuel cells have been around for almost 200 years, they are used by humans at the bottom of the oceans and outside of earth’s atmosphere; but only now with efficient processes to create green hydrogen, they are becoming economically viable solution to help us reach the 2050 Net Zero Goal.

