Hydrogen is fast becoming an important part of the worldwide energy mix in both transport and stationary applications (such as a HydroGenesis system). Storage of large amounts of energy generated from intermittent renewable sources is driving a tightening and specification of safety standards.
In 2015, a European consortium funded by European Union Fuel Cells and Hydrogen Joint Undertaking, published a detailed report titled Hyindoor on the basics of hydrogen safety. The report has been adopted and disseminated by various international bodies such as the ISO (International Organisation for Standardisation) and country specific regulatory institutions, such as the Health and Safety Executive (HSE.gov.uk) in the UK. The report details a lot of risks and raises awareness of the reasons why strict adherence to safety is important. The main conveyed message is that simple processes can be followed to make the handling of hydrogen safe; points relevant to stationary storage can be summarised in the extracted points below:
- Consider whether it is necessary to house the hydrogen system within a room or enclosure, or whether it could be relocated outdoors where accidental leak would less likely lead to accumulation of hydrogen in flammable concentrations due to better ventilation.
- Reduction of hydrogen supply pipeline diameter and operational pressure to the minimum required to satisfy technological requirements for mass flow rate.
- Install various levels of ventilation to prevent build up in high-level areas.
The report advises that use of hydrogen in a confined environment requires detailed assessment of hazards and associated risks, including potential risk prevention and mitigation features. In short, the above three points detail that as long as the Hydrogen is stored in a correct manner, with acceptable pressure limitations and key mitigation and monitoring, the safety of hydrogen can be managed to a mass-acceptable, and more importantly mass-adoptable degree. Hydrogen has many properties that actually assist in the safety and prevention of danger, this article will attempt to summarise those reasons.
When people think about hydrogen, among the first thoughts is normally the 1937 Hindenburg disaster. This disaster imprinted a graphic memory on the world that hydrogen is dangerous and explosive, and it is in certain situations. Hydrogen was used in the Hindenburg as it is the lightest gas in the entire Universe (though the airship was initially designed to be fuelled by the more inert helium, but that is a different story). One litre of hydrogen weighs only 90 milligrams under normal atmospheric pressure, meaning it is eleven times lighter than the air we breathe. If released in unrestricted air, hydrogen disperses vertically at 50m/s (112 mph); a good way to visualise this speed with by thinking of a golf ball being driven from a tee.
The Hindenburg’s hydrogen was unpressurised so it was following the development of a ‘slow leak’ that prevented the gas escaping rapidly. A leak from a pressurised vessel would have caused the gas to escape away and not allow the hydrogen to mix with oxygen within the container, which is required for combustion. Explosions and a fire are not possible if pure hydrogen is properly contained in pressure vessels as hydrogen can only ignite around a flame and if there is a greater than 4% v/v hydrogen, which is the lower explosive limit (LEL), up to as much as 75% v/v, which is the upper explosive limit (UEL)*.


Zeppelin the Hindenburg on fire at the mooring mast of Lakehurst (United States of America) 6 May 1937. Ballast water is thrown down. Exit airships.

Due to its lightness, hydrogen will concentrate in elevated regions of an enclosed space, whereas other well-used gases, dependent upon their relative mass, will concentrate at ground level or at low elevation (such as LPG or Natural Gas). The general advice for hydrogen systems is to maintain a good level of ventilation (if outside storage is not possible) and by locating all potential sources of ignition lower than the level of the equipment from which hydrogen may leak and accumulate to whilst ensuring adequate ventilation and safe discharge.
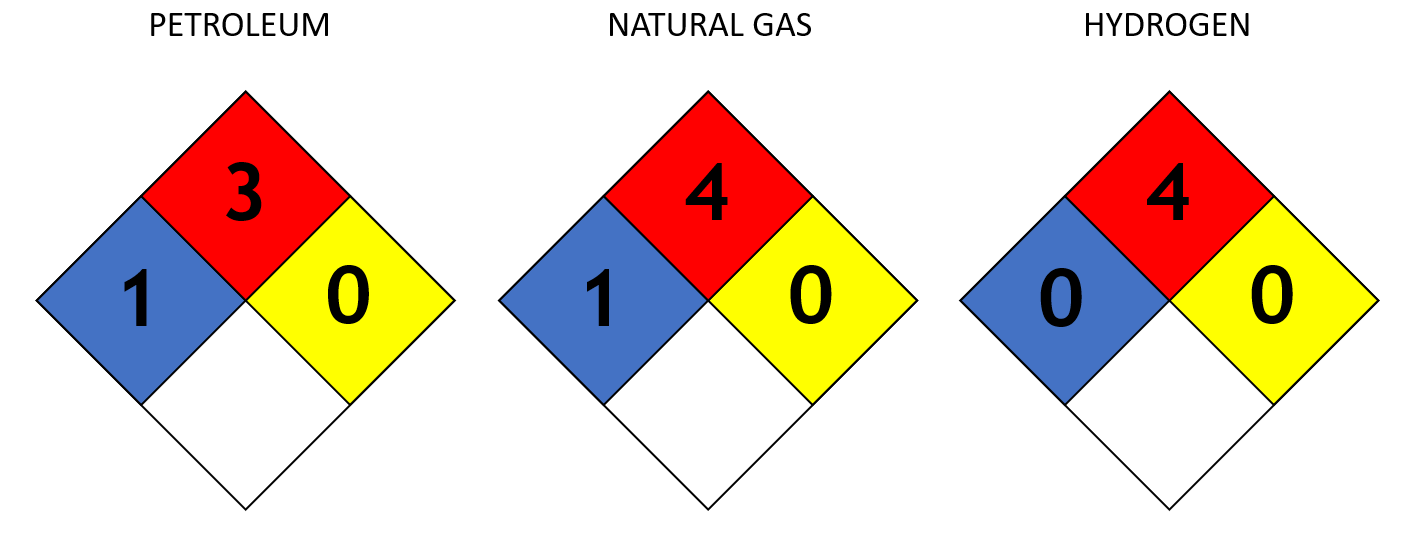
Above is a picture of the "Safety Square" or "Fire Diamond" which is used by emergency teams to quickly and easily identify the risks posed by hazardous materials during the initial stages of an emergency response. The top three coloured spaces have a number ranging from of 0 to 4 (0 being of no concern to 4 being extremely high risk) identifying the Flammability (RED), Health Hazard (BLUE), and Reactivity (YELLOW) with the white space reserved for corrosivity or special toxicity listings other materials. The left diamond above shows the rating for petroleum, the middle natural gas and the right hydrogen; hydrogen has the highest possible flammability rating but also has the lowest possible rating for health hazard and reactivity, petrol and natural gas are both rated as more dangerous.
Hydrogen is a colourless, odourless gas that is undetectable by our natural human senses. Adding in an odorant, as is done with natural gas in the national grid to help us identify leaks, has been widely considered but deemed unsuitable for most hydrogen systems due to the damage the additives can cause to fuel cell membrane catalysts. Also the size of the odorant molecules are much larger than the hydrogen molecules, so will not be detectable in the slow and most dangerous of hydrogen leaks.
The pressure at which hydrogen is being stored is a critical safety factor that needs careful mitigation and safety systems. 1kg of hydrogen will require a space of approximately 11 sq. M if uncompressed at atmospheric conditions. For a seasonal stationary storage application that requires approx. 25kg for a domestic system and >100kg+ for a commercial setting, the gas needs to be compressed to make the application viable. There are a number of storage solutions currently on the market and in development for hydrogen with pressure ratings from 35 bar to 1000 bar. These storage solutions need to be specialised as hydrogen can cause embrittlement of high strength steels, titanium alloys and aluminium alloys with cracking and catastrophic failure of the metals at low stress levels, but this will be covered in a future article on the “Four Different Types of Hydrogen Cylinders”.
With low to zero risk of explosion due to containing pure hydrogen, the high pressure becomes the main safety concern with hydrogen cylinders. A vessel releasing pressure through a small opening has the ability to act like a missile, imagine a balloon being let go, except that is not a piece of rubber flying through the air… This is where hydrogen safety standards discussed in the Hyindoor report become key, such as the minimum length of high-pressure pipe work, from the pressure source to the regulator, as well as a number of pressure release valves at key interfaces to vent hydrogen when necessary, into a safe, open space. Standard and well understood physics are needed to eliminate any blast pressure related incidents, but they should not be trivialised.
Key characteristics of hydrogen when looking from a safety perspective:
- Hydrogen gas has a very low viscosity so creates a challenge in leak prevention. Hydrogen-suitable sealing interfaces with welded not threaded joints, along with appropriate hydrogen-rated components and limited interference with the system once commissioned will significantly reduce the leak potential.
- The energy necessary to initiate a hydrogen/air explosion is very small. The ignition energy for a 2:1 hydrogen/oxygen mixture is only about 0.02 mJ. This is less than one tenth that of other fuels such as methane, LPG or petrol. Even very small sparks, such as those produced by wearing certain types of clothing, are capable of igniting hydrogen/air mixtures and causing an explosion. Therefore, detection devices, active ventilation systems and shut-off valves are necessary to remove any risk of ignition
- Hydrogen burns with an invisible flame making it difficult to detect a hydrogen fire. This low emissivity of hydrogen flames reduces the heat transfer by radiation to other objects in close proximity, thus reducing the risks of secondary ignition. Similarly, the speed at which hydrogen burns means that surrounding objects and materials are exposed for less time when compared to similar volumes of other flammable gases; so again, there is less at risk of secondary ignition.
Hydrogen is less dangerous than natural gas and petrol but due to our familiarity and strict safety codes when working with them, the general world population are comfortable using and operating machines combusting them. Hydrogen may have been tarnished early-on in its life as an energy vector by the Zeppelin airships, but the benefits it brings to a world demanding clean fuel, when coupled with risk reduction and mitigation, means the hydrogen-fuelled Earth is coming.
* Volume concentration of a gas is expressed as % v/v, which stands for volume per volume.


